Home > Blog > Pharma Compliance: Flammable Storage Cabinet Solutions for Pharmaceutical Manufacturing
-
 Sarah
Hi there! Welcome to my shop. Let me know if you have any questions.
Sarah
Hi there! Welcome to my shop. Let me know if you have any questions.
Your message has exceeded the limit.

Pharma Compliance: Flammable Storage Cabinet Solutions for Pharmaceutical Manufacturing
2025-11-06 13:40:31
Pharmaceutical manufacturing facilities operate under some of the most stringent regulatory requirements in any industry, making proper flammable liquid storage essential for maintaining compliance and ensuring product safety. The implementation of appropriate flammable storage cabinet solutions represents a critical component of pharmaceutical compliance programs that protect both workers and product integrity. This comprehensive guide explores the specific requirements for flammable storage in pharmaceutical manufacturing and how proper implementation supports regulatory compliance and operational excellence.

Understanding Pharmaceutical Regulatory Requirements
Pharmaceutical manufacturing must comply with multiple regulatory frameworks including FDA Current Good Manufacturing Practices (cGMP), OSHA standards, and EPA regulations. These regulations establish specific requirements for chemical storage, handling, and documentation that directly impact flammable liquid storage cabinet selection and implementation. Pharmaceutical facilities must understand these various requirements to ensure comprehensive compliance.
The FDA’s cGMP requirements extend to all aspects of pharmaceutical operations, including chemical storage and handling. Flammable storage cabinets must support compliance with these requirements through proper construction, organization, and documentation. Additionally, pharmaceutical facilities must comply with OSHA’s Process Safety Management (PSM) standard when handling certain quantities of flammable liquids, requiring additional safety measures and documentation.
Chemical Segregation and Compatibility
Pharmaceutical manufacturing involves numerous chemicals that may be incompatible with each other, requiring careful segregation and storage planning. Flammable storage cabinets must support proper chemical segregation based on compatibility charts and regulatory requirements. Different pharmaceutical processes may require specific chemical combinations that must be stored separately to prevent dangerous reactions.
Chemical compatibility considerations extend to both the chemicals themselves and their containers. Some pharmaceutical processes may use chemicals that can react with cabinet materials or other stored substances. Pharmaceutical facilities should conduct thorough compatibility assessments before implementing storage solutions to ensure that all chemicals can be stored safely without risk of reaction or contamination.
Cleanroom Compatibility and Contamination Control

Pharmaceutical manufacturing often occurs in cleanroom environments where contamination control is critical for product quality and patient safety. Flammable storage cabinets used in or near cleanrooms must meet specific cleanliness standards and prevent contamination of both stored chemicals and the manufacturing environment. Cabinet construction materials should be non-shedding and easy to clean to maintain cleanroom integrity.
Cleanroom compatibility considerations extend to cabinet placement and integration with cleanroom protocols. Some pharmaceutical facilities implement anterooms or gowning areas where flammable storage cabinets are positioned to maintain cleanroom integrity while providing necessary chemical access. The integration of storage cabinets with cleanroom management systems ensures comprehensive contamination control and regulatory compliance.
Documentation and Traceability
Pharmaceutical manufacturing requires comprehensive documentation of all processes and materials, including flammable liquid storage and usage. Storage cabinets should support documentation requirements through organized storage that facilitates inventory tracking, usage recording, and regulatory reporting. Some pharmaceutical facilities implement digital inventory management systems that integrate with storage cabinets to provide real-time data on chemical usage and availability.
Traceability considerations extend to batch tracking and quality control procedures. Flammable liquids used in pharmaceutical manufacturing may be associated with specific production batches, requiring careful tracking and documentation. Storage cabinets that support organized segregation and clear labeling facilitate accurate documentation and traceability throughout the manufacturing process.
Temperature and Humidity Control
Some pharmaceutical processes require flammable liquids to be stored under specific temperature and humidity conditions to maintain chemical stability and product quality. Storage cabinets may need to incorporate climate control features that maintain these conditions while providing necessary safety protection. Temperature and humidity monitoring systems should be integrated with cabinet storage to ensure proper storage conditions.

Environmental control considerations extend to the integration of storage cabinets with the facility’s HVAC and environmental monitoring systems. Some pharmaceutical facilities implement specialized environmental zones where flammable storage cabinets are positioned to maintain optimal storage conditions. The integration of environmental control with safety features ensures comprehensive protection of both chemicals and pharmaceutical products.
Security and Access Control
Pharmaceutical manufacturing facilities often handle valuable or controlled substances that require additional security measures beyond standard safety requirements. Flammable storage cabinets should incorporate security features that prevent unauthorized access while maintaining accessibility for authorized personnel. This balance between security and accessibility is particularly important in pharmaceutical facilities with strict access control requirements.
Security considerations may include electronic access control systems, biometric authentication, or integration with the facility’s security infrastructure. Some pharmaceutical facilities implement inventory management systems that track chemical usage and access, providing additional security and accountability. The selected security features should align with the facility’s overall security policies while supporting efficient manufacturing operations.
Validation and Qualification Procedures
Pharmaceutical equipment and systems must undergo rigorous validation and qualification processes to ensure they meet regulatory requirements and perform as intended. Flammable storage cabinets should be included in these validation processes, with documentation of installation qualification, operational qualification, and performance qualification. This validation ensures that cabinets meet all specified requirements and support compliance.
Validation considerations extend to ongoing performance monitoring and requalification procedures. Pharmaceutical facilities should establish schedules for periodic revalidation of storage systems to ensure continued compliance and performance. Documentation of all validation activities creates a comprehensive record that demonstrates regulatory compliance and supports continuous improvement of storage systems.
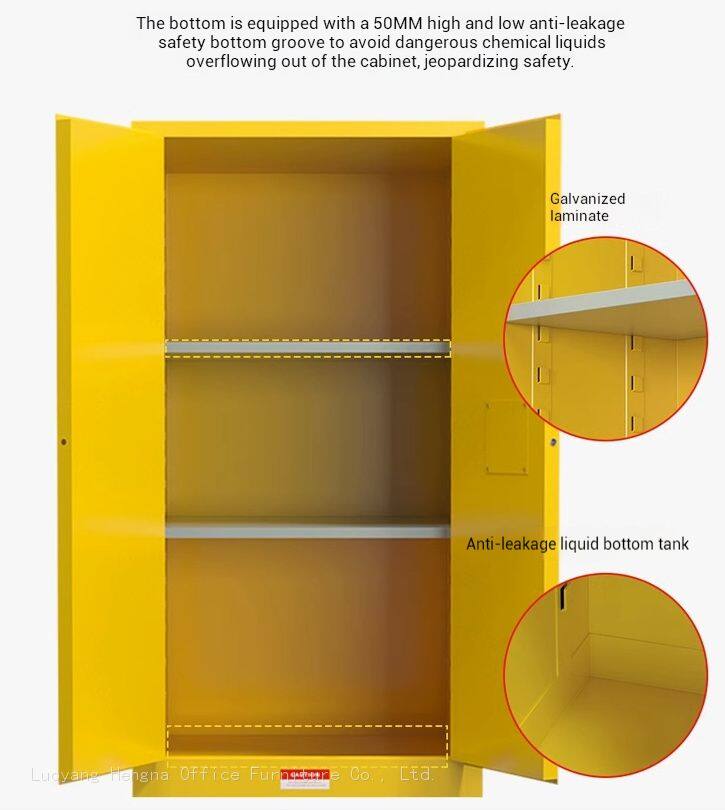
Integration with Quality Management Systems
Pharmaceutical manufacturing operates under comprehensive quality management systems that govern all aspects of production and facility operations. Flammable storage cabinets should integrate with these quality systems through proper documentation, standard operating procedures, and quality control measures. The integration ensures that storage practices support overall quality objectives and regulatory compliance.
Quality management integration benefits extend to supporting various quality assurance activities including audits, inspections, and continuous improvement initiatives. Organized storage systems facilitate quality audits by providing clear evidence of proper chemical handling and storage practices. Some pharmaceutical facilities use storage cabinet organization as part of their quality training programs, demonstrating commitment to quality and safety excellence.
Tags: Pharma Compliance, Flammable Storage Cabinet Solutions, Pharmaceutical Manufacturing

